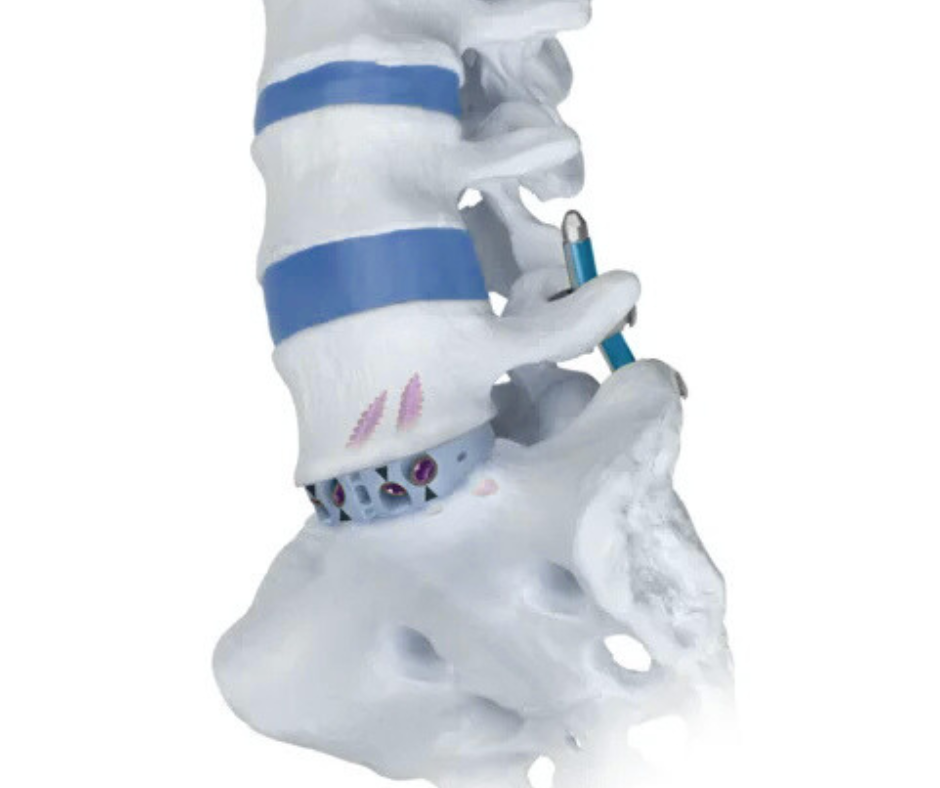
Introduction
On March 11, 2025, Globus Medical introduced two revolutionary products for anterior lumbar interbody fusion (ALIF): the COHERE™ ALIF Spacer, the first porous PEEK interbody spacer specifically designed for ALIF, and the Modulus™ ALIF Blades, developed to optimize immediate fixation after implant insertion. Both technologies are part of Globus Medical’s Advanced Materials Science™ (AMS) line, aimed at improving osteointegration and surgical efficiency.
1. Context and Rationale for Development
Traditionally, solid PEEK interbody spacers offered rigidity and good radiographic visibility but had limitations in bone integration, often requiring additional graft materials to ensure fusion. By incorporating a porous surface, the
COHERE™ ALIF Spacer aims to address this issue by allowing bone growth through the implant, reducing the risk of pseudarthrosis and promoting a firmer, more stable fusion.
Meanwhile, the Modulus™ ALIF Blades extend the capabilities of the Modulus™ ALIF system by offering, through low-profile anchoring blades, immediate fixation after spacer placement, reducing the number of surgical steps and instrument handling in the operative field.
2. Technical Features of the COHERE™ ALIF Spacer
2.1 Material and Porous Technology
- Material: Made of Globus Medical’s patented porous PEEK, it combines the characteristic rigidity and radiolucency of PEEK with an architecture designed to promote osteointegration. Preclinical studies show that this optimized porosity fosters faster bone growth and superior biomechanical strength compared to traditional solid PEEK.
- The porous surface provides a micro-structure that facilitates osteoblast adhesion, allowing bone to grow “into” the implant and create a firmer seal between the adjacent vertebra and the device.
2.2 Geometric Design and Biomechanics
- Its geometry replicates the natural architecture of trabecular bone, maintaining consistent porosity levels under loads similar to those of the lumbar spine, preventing deformation or delamination during insertion.
- Spacer dimensions and lordotic angle are offered in multiple options to suit different lumbar anatomies, maximizing disc height restoration and sagittal alignment.
2.3 Radiographic Visibility
By retaining PEEK’s inherent radiolucency, COHERE™ allows clear postoperative monitoring via X-rays and computed tomography scans, avoiding metallic artifacts and facilitating assessment of bone fusion.
3. Clinical Advantages and Potential Impact
3.1 Improved Osteointegration and Early Fusion
COHERE™’s porosity increases the bone-contact surface area, which may translate into higher successful fusion rates and lower risk of complications associated with pseudarthrosis. According to Frank Phillips, MD (Rush University Medical Center), “the combination of rigidity, radiolucency, and potential osteointegration of COHERE™ provides a significant clinical benefit in ALIF surgery.”
3.2 Reduced Surgical Steps and Operating Time
The introduction of the Modulus™ ALIF Blades optimizes fixation by allowing anchoring blades to be inserted immediately after spacer placement, securing the device to the adjacent vertebra without the need to switch instruments or reorganize the operative field. This design can reduce total surgery time and minimize the risk of vascular complications by limiting exposure and manipulation of soft tissues.
3.3 Simplified Use of Bone Graft
By promoting fusion without relying on additional grafts, COHERE™ may decrease the need to harvest autologous graft or use biomaterial packs, reducing indirect costs and morbidity associated with graft harvesting.
3.4 More Reliable Postoperative Monitoring
The material’s radiolucency allows evaluation of fusion progression without metallic interference, facilitating early detection of potential migration or integration failures, and ensuring safer clinical follow-up.
4. Market Impact and Initial Adoption
Concurrent coverage in high-impact outlets such as Becker’s Spine Review, Surgical Robotics Technology, Nasdaq, and GlobeNewswire highlights global interest in this technology. In spine surgery environments where efficiency and fusion reliability are critical, COHERE™ and its associated Blades are anticipated to gain rapid acceptance due to:
- Technological Differentiation: Being the first porous PEEK ALIF spacer on the market provides a competitive advantage over smooth or titanium-coated spacers.
- Potential Reduction in Revisions: By improving fusion rates and minimizing vascular complications, it may decrease the need for revision surgeries, positively impacting patient satisfaction and optimizing hospital resources.
- Strategic Value for Globus Medical: It strengthens the AMS line and solidifies the company’s position in the advanced lumbar spine devices segment.
5. Considerations for the Spine Surgeon
1. Surgical Technique Training
Given the novel porous surface, attending workshops or training sessions organized by Globus Medical is recommended to learn the proper insertion technique, avoiding risks of delamination or improper spacer placement.
2. Documentation and Outcome Tracking
It is essential to document short- and long-term outcomes (fusion percentage, pain scales, complications) to confirm the benefits observed in preclinical studies and initial patient series.
3. Cost-Benefit Evaluation
In centers where revisions due to fusion failures represent a high expense, investing in porous technology may lead to medium-term savings.
Conclusion
The COHERE™ ALIF Spacer and Modulus™ ALIF Blades represent a disruptive advance in ALIF surgery by integrating porous PEEK technology that promotes more reliable fusion and optimizing immediate fixation after insertion. The extensive coverage in specialized and financial media indicates that this innovation has captured the attention of both the medical community and the market. For spine surgeons, adopting these solutions means improving clinical outcomes, reducing associated morbidity, and optimizing the use of operating room resources.
References
Globus Medical extends versatility of Advanced Materials Science™ anterior interbody portfolio with launch of COHERE™ ALIF Spacer and Modulus™ ALIF Blades. GlobeNewswire, March 11, 2025.
Globus Medical launches COHERE™ ALIF Spacer and Modulus™ ALIF Blades to enhance anterior lumbar spine surgery solutions. Nasdaq, March 11, 2025.
Globus Medical expands spine portfolio with 2 commercial launches. Becker’s Spine Review, March 2025.
Globus Medical launches COHERE™ ALIF Spacer and Modulus™ ALIF Blades. Surgical Robotics Technology, March 2025.
Modulus™ ALIF Blades. Globus Medical.
Cohere™ ALIF. Globus Medical.